One important benefit of Bayesian statistics is that you can
provide relative support for the null hypothesis. When the null hypothesis is
true, p-values will forever randomly
wander between 0 and 1, but a Bayes factor has consistency (Rouder,
Speckman, Sun, Morey, & Iverson, 2009), which means that as the
sample size increases, the Bayes Factor will tell you which of two hypotheses
has received more support.
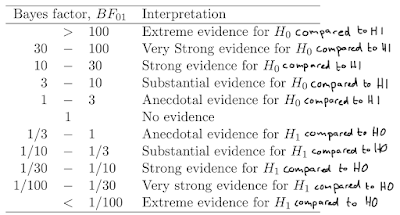
Bayes Factors can express relative evidence for the null hypothesis (H0) compared
to the alternative hypothesis (H1), referred to as a BF01, or relative evidence for H1 compared to H0 (a BF10).
A BF01 > 3 is sometimes referred to as substantial evidence for
H0 (see Wagenmakers,
Wetzels, Borsboom, & Van Der Maas, 2011), but what they really mean is
substantial evidence for H0 compared to
H1.
Table 1 from
Wagenmakers et al., 2011, #fixedthatforyou
Since a Bayes Factor provides relative support for one over another hypothesis, the
choice for an alternative hypothesis is important. In Neyman-Pearson Frequentist approaches, researchers have to specify the alternative hypothesis
to be able to calculate the power of their test. For example, let’s assume a
researcher no idea what to expect, and decided to use the average effect size
in psychology of d = 0.43 as the
expected effect when the alternative hypothesis is true. It then becomes easy
to calculate that you need 115 participants in each group of your experiment,
if you plan to perform a two-sided independent t-test with an alpha of 0.05, and you want to have 90% power.
When calculating the Bayes Factor, the alternative is
specified through the r-scale. This is a scale for the Cauchy prior, which is
chosen in such a way that the researcher expects there is a 50% chance of
observing an absolute effect larger than the scale value chosen. As Rouder and
colleagues say: “A well-known and attractive alternative to placing priors on
the mean µ is to place them on effect size, where effect size is denoted by δ
and given as δ = µ/σ. Thus, the researcher above would choose an r-scale of
0.43.
The default Bayesian t-test
originally used a r-scale of 1 (a very large effect), but the updated default
test uses a r-scale of 0.707. This means that whenever you perform a study
where you calculate the default Bayes Factor, and find a BF10 < 1/3,
you have observed support for the null-hypothesis, relative to an alternative
hypothesis of an effect of d = 0.707.
Let’s assume the default Bayesian
t-test answers a question
you are interested in, and you want to know whether your data is stronger
support for a
d = 0 compared to a
d = 0.707. You know that to have 90% power to
observe a
d = 0.707 in an independent
t-test,
you need 44 participants in each condition, and you have heard people recommend
to use at least 50 participants in each condition, so you set out to collect 50 participants in each condition. You
are also completely fine with a null-result: You have submitted your study to
a journal that accepts pre-registered reports, and the reviewers there agreed the
data will be interesting whether they support the null hypothesis or the
alternative hypothesis. What is the probability you will observe support for H0
relative to H1 (see
this paper by Felix Schonbrodt and colleagues for similar Frequentist calculations of Bayes Factors)?
There are no Bayesian power calculators (yet, as far as I know). Bayes Factors
express the relative evidence in your data, and you don’t need a threshold to
interpret a Bayes Factor. In principle, any BF
10 < 1 provides
stronger support for the null hypothesis than for the alternative hypothesis. But
no one will be convinced if you argue for the null hypothesis based on a BF
10
= 0.98, and it’s practical to have some sort of agreed upon threshold where we
all agree data can be interpreted as ‘support’ – hence tables with Bayes Factor
thresholds as the one reported in Wagenmakers et al, 2011 (see above). So what are the chances
of actually observing relative support for the null hypothesis, compare to an
alternative hypothesis of
d = 0.707, with 50 participants in each condition,
when you want a
BF10 < 1/3?
Using the R code below, you can easily calculate this
probability yourself. It also provides the probability of finding support for
the alternative hypothesis. This means you can run the simulations twice, once
setting the true effect size to 0, once setting the true effect size to 0.707,
and see whether your sample size is large enough to get what you want from the
data you plan to collect.Note you need quite some simulations to be accurate, and this takes minutes)
Running these simulations, we see there’s about a 69%
probability of finding support for the null-hypothesis with a BF
10
< 1/3 if the true effect size is 0. In the histogram of Bayes Factors below, this is the distribution to the left of the left vertical red line. Approximately 1.6% of the Bayes Factors give misleading evidence (a BF > 3, to the right of the right vertical red line) and around 30% of the Bayes Factors are inconclusive evidence.
The second set of simulations reveals there’s approximately a 85% chance of finding a
BF
10 > 3 when the true effect size is 0.707. Based on these data,
and your explicit interest in providing support for the null-hypothesis, you
might want to collect some more participants.
Let’s look at some other scenarios. Imagine that in the
previous situation, the true effect size was d = 0.43. It turns out that 13% of
our experiments will find relative support for the null-hypothesis, 38% of the
tests will find relative support for the alternative hypothesis, and 48% of the
tests will be inconclusive, with a Bayes Factor between 1/3 and 3. Luckily,
with Bayesian statistics you can continue collecting data, but since no one has
unlimited resources, it’s important to know whether it’s feasible to collect
sufficient data to answer your question in the first place. For example, in the default Bayesian t-test, don’t bother trying to find ‘strong
evidence’ (BF < 1/10) for the null if you are planning to collect less than 200 participants. When the null is really true, and you plan
to collect data from 100 to 200 participants in each condition, you’ll never find the evidence you are looking
for. With 300 participants in each condition, you’ll start to get around 33%
probability of finding what you want, indicating a threshold of 10 might be unreasonable
for single studies, and more suitable for meta-analyses.
The simulations also show that the probability of an
incorrect conclusion (observing support for H0 when H1 is true, or support for
H1 when H0 is true) is very low. Indeed, it’s so low, that we might want to
reconsider used a Bayes Factor of 3 as a threshold.
For example, if we set a threshold as low as 1.2, instead of
3, the simulations show that, when the null-hypothesis is true, approximately 5% of
the Bayes Factors we observe will provide relative support for the alternative
hypothesis, and over 90% of the Bayes Factors will provide relative support for
the null hypothesis, with 50 participants in each condition (the remainder, 5% fall in the small inconclusive evidence area). This may not be ‘substantial
evidence’ but you won’t be wrong very often, in the long run, when you decide
to accept the null hypothesis over the alternative hypothesis of d = 0.707 when
you collect 50 participants in each condition.
You may not like the use of a cut-off value to indicate ‘relative
support’ as proposed by some Bayesians (Dienes,
2016; Wagenmakers et al., 2011). I think these cut-offs are
most important when planning an experiment. It makes sense to aim for support most
people will find convincing. When the data are in hand, you can interpret it
any way you like. If you agree, then important questions are which cut-off
values are acceptable under which circumstances? Can we develop formulas for
Bayesian power analysis instead of having to rely on simulations?
I learned something by playing around with these simulations,
and I expect others moving to Bayes Factors will have similar questions as I tried to examine here. Power analysis is Frequentist concept, and it might not be close to the heart of Bayesians, but I expect it meets a future need of researchers
switching to Bayesian statistics, especially when they want to design studies
that provide support for the null.
Dienes, Z.
(2016). How Bayes factors change scientific practice. Journal of
Mathematical Psychology. http://doi.org/10.1016/j.jmp.2015.10.003
Rouder, J. N., Speckman, P. L., Sun, D., Morey, R. D., & Iverson,
G. (2009). Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review, 16(2), 225–237.
http://doi.org/10.3758/PBR.16.2.225
Wagenmakers, E.-J.,
Wetzels, R., Borsboom, D., & Van Der Maas, H. L. (2011). Why
psychologists must change the way they analyze their data: the case of psi:
comment on Bem (2011). Retrieved from
http://psycnet.apa.org/journals/psp/100/3/426/